Where Do Lithium-Ion Batteries Stand in 2020?
The rise of small, portable electronics has demanded the concurrent development of powerful battery technology. Since the 1990s, lithium-ion batteries (Li-ion batteries or LIBs) have emerged as a frontrunner in the hardware arms race—usurping their nickel-based counterparts.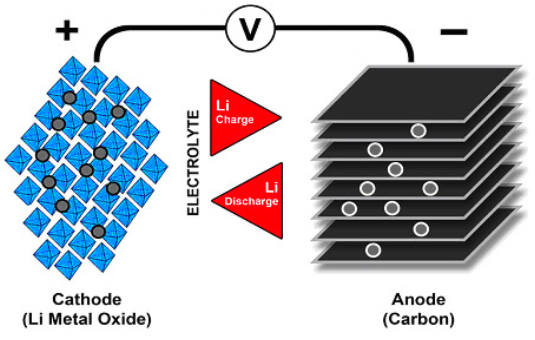
Flow of ions in a Li-ion battery. Image used courtesy of Battery University
Development cannot stagnate, however. We’ll explore what LIBS brings to the table, and why EEs working with R&D battery technology shouldn’t be complacent with LIB progress quite yet.
The Advantages of Lithium-Ion
Compared to metallic alternatives, lithium-ion is more stable during operation and while charging. Despite their lithium metal predecessors’ higher density, LIBs remain twice as energy-dense as nickel-cadmium batteries. They also have the following favorable characteristics:
- Single-cell construction and consolidation
- No memory or required charge scheduling for maintenance
- Slow discharge during rest
- Relatively-safe disposal
- Customizable cell configurations for diverse applications
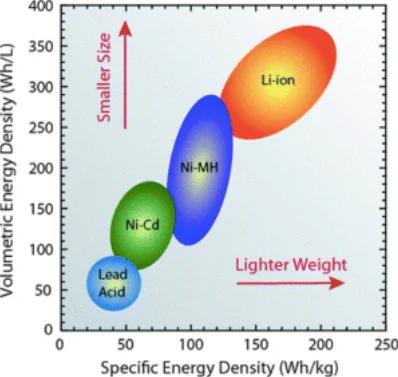
Li-ion batteries outrank many other battery types in terms of specific energy density and volumetric energy density. Image used courtesy of Roberta A. DiLeo, Rochester Institute of Technology and the Clean Energy Institute
Li-ion technology generally retains an advantageous cost-energy ratio, although certain types of lithium-ion cells (prismatic slim) are more expensive. These batteries are also easy to replace while enjoying a long shelf life.
Stanford Suggests Solid Materials to Replace Liquid Electrolytes
A typical lithium-ion battery is composed of two electrodes with liquid electrolytes filling the space between. This liquid is volatile; punctures or shorts may cause ignition. Some manufacturers’ designs have been particularly susceptible. Note that a typical LIB includes a separator, which keeps the electrodes spaced apart while allowing energy transfer.
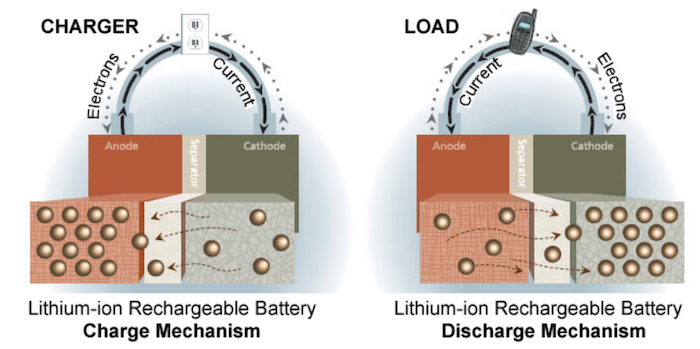
Diagram of a lithium-ion cell, including a separator and an ion flow between electrodes. Image used courtesy of Battery University
You might recall the spontaneous combustion debacle that plagued Samsung’s Galaxy Note 7; these device fires were ultimately caused by crimping, separator damage, and short-circuiting.
Stanford University researchers now suggest that solid materials could be worthy replacements for liquid electrolytes. They’re also more cost-effective.
Lithium, boron, and sulfur have risen to the top (thanks to machine learning screenings) as viable materials. Solids can withstand stress and resist breaking down for many more cycles, supporting the notion that solids can be conductive for much longer periods of time. Short-circuiting is also much less common.
The biggest challenges will be securing manufacturing pipelines and bridging the conductivity gap between liquids and solids.
South Dakota State University Champions Lithium Metal
It’s said that lithium metal is a holy grail in battery research. However, the long-term reliability of this material is questionable when used for anodes. The foil forms sharpened protrusions called dendrites over time. These dendrites can internally puncture the separator, thus causing shorts and fires.
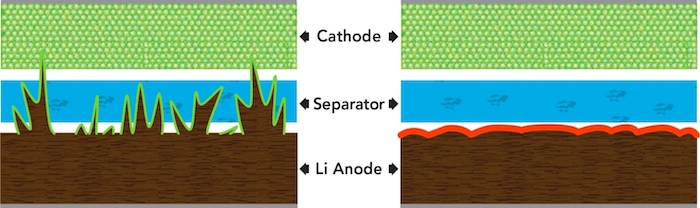
The dendrites (pictured on the left) could break through separator material and cause short circuits and fires. Image used courtesy of Dean Sigler
What if we could stop dendrite growth in its tracks? Scientists at SDSU suggest that a new lithium-nitride coating between the anode and separator can do just that—negating the pitfalls of uneven lithium metal distribution.
The plasma-processed coating also promotes prolonged ionic conductivity during the battery’s lifespan. Lithium-metal batteries will accordingly enjoy greater popularity and even mechanical strength. They’ll also retain their capacity more effectively.
The Future of Lithium-Ion and its Cousins
It’s encouraging to see how far lithium-ion batteries have come since their inception. While the technology has achieved a degree of maturation, researchers are still finding ways to improve current technologies. Though many may suggest that battery development has slowed, the world’s foremost companies and universities are proving otherwise. We’re also finding new ways to make LIBs more economical during production.
That’s not to say that lithium-ion technology is the end-all-be-all. Global lithium reserves face a potential threat if development and consumption accelerate. Safety is vastly improved, and while battery failures are rare (under one in a million, according to Battery University), those failures can cause bodily or property damage.










