How to Choose Batteries
Batteries are portable storehouses of energy. They power our headlamps, lanterns, GPS devices, cameras, music players and more. The ideal battery will give you a balance of long duration, high performance, fair cost and low environmental impact. In order to get that, you have to know what you’re looking for, which can be tough when you start digging into details about electrodes, cathodes and different metal types.
In this guide, we walk you through the options and include the pros and cons of different battery types as they relate specifically to outdoor users, like hikers, bikers, skiers and climbers.
Tips for Choosing Batteries:
- Figure out what size batteries you need: This is simple. If your gadget runs on AAA batteries, then that’s what you need. You can look on the device itself for an indication of what battery size it takes, or consult the instruction manual.
- Decide between single-use or rechargeable batteries: Single-use batteries are cheaper upfront and have an excellent shelf life, but rechargeables can be used again and again, making them ultimately the more cost-effective choice.
- Get the right type of battery: Understanding how batteries work and knowing how alkaline differs from lithium and NiMH from lithium-ion will help you pick the best battery for your application.
Figure Out What Size Batteries You Need
You don’t need to know much about batteries to get the right size for your device. Figuring it out can be as easy as looking at the batteries currently in your device and replacing them with the same size (i.e. if there are AAA batteries in there, then that’s what size you need to buy). If you don’t already have batteries installed, look on the device for some indication or check the instruction manual.
If you want to know a little more about battery sizes, here’s a quick primer:
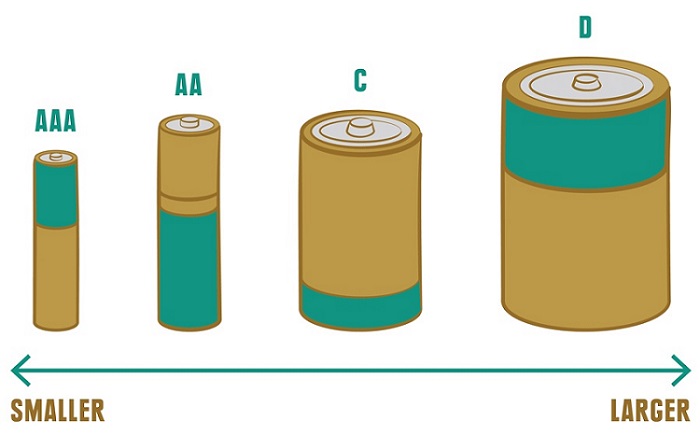
- You’re probably familiar with AAA, AA, C and D batteries. Those letters are indicators of size. Basically, the farther you get through the alphabet, the larger the battery (e.g. D is bigger than C). When you see a letter used more than once (eg. AA, AAA), the more times it’s used, the smaller the battery (eg. AAA is smaller than AA).
Choose Single-Use or Rechargeable
If you’re shopping for common cylindrical batteries, like AAA, AA, C or D, you have the option of buying single-use batteries or rechargeable batteries (coin-cell batteries, like CR2032, are single-use only). Both have advantages and disadvantages; Here’s a quick look at those:
Single-use batteries: These are what they sound like. When they run out of juice, you need to dispose of them (to find battery recycling options near you, visit call 2Recycle.org. The two main types of single-use batteries are alkaline and lithium.
Pros:
- Cheaper upfront cost than rechargeable batteries.
- Very low self-discharge rate (power loss when not in use) for a long shelf life.
- Widely available.
Cons:
- Require disposal after fully discharged.
Rechargeable Batteries: These batteries are built to be recharged over and over again, in some cases up to 500 times or more. The two main types of rechargeable batteries are nickel-metal hydride and lithium-ion.
Pros:
- Because they’re rechargeable, they generate less waste than single-use batteries.
- They offer better long-term value than single-use batteries (the more you use them, the cheaper they get).
Cons:
- More expensive upfront cost than single-use batteries.
Get the Right Type of Battery
Once you’ve settled on the battery size and decided between single-use and rechargeable, you may find it helpful to understand a bit more about the different types of batteries. With a basic understanding of how batteries work and what’s inside them, you can make more informed decisions about the right type of batteries for your needs.
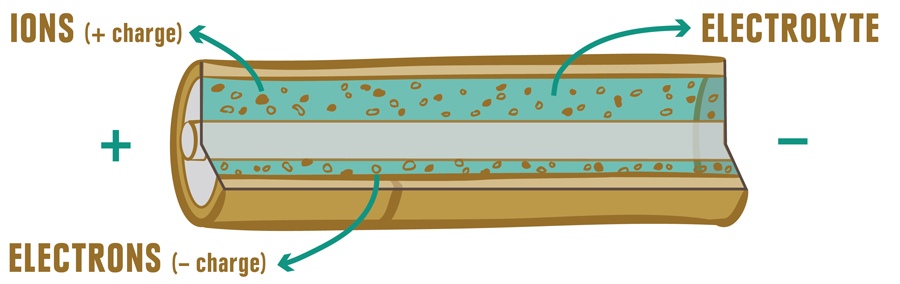
Battery basics: Common batteries, such as AAA, AA, C and D, have positive and negative terminals and two internal layers called electrodes that include a cathode (which transports a positive charge) and an anode (to carry a negative charge). All batteries also have some type of electrolyte—a substance that conducts electricity (a flow of electrons) between the battery’s terminals. When you put a battery in a device, like a headlamp, the electrolyte, cathode and anode interact and a chemical reaction (basically oxidation) occurs. Ions (positively charged) and electrons (negatively charged) flow through the electrolyte, exit via the negative terminal and enable your device to function.
Over time, a battery's internal chemicals begin to degrade and interaction diminishes. Eventually they can no longer retain a charge and are considered “dead.”
The mix of chemicals in a battery aims to provide some combination of the four holy grails of the elusive "ideal" battery—long life, high performance, reasonable cost and low environmental impact. Here’s a closer look at the most common options available for single-use and rechargeable batteries:\
Single-Use Batteries
Single-Use Alkaline Batteries
The most commonly used battery of all is an alkaline battery (meaning it contains an alkaline electrolyte, usually potassium hydroxide).
Best use: "Low-drain" devices such as LED headlamps, LED flashlights, toys, remote control devices, clocks and radios, and even moderate-drain items such as lights using incandescent bulbs. Alkaline batteries can be used in high-drain devices (digital cameras, for instance), though their life expectancy will be sharply reduced. Why? Even though alkalines have high initial energy capacity, high-drain devices exert such a substantial draw that energy swiftly gets drawn down.
Pros:
- Moderately priced
- Widely available
Cons:
- Perpetual cycle of use-disposal-replacement. Can possibly be recycled, but most wind up in landfills.
Battery Tips
To get the most out of your batteries, follow these tips when possible:
- All batteries, even those designed to handle extreme temperatures, can experience a decline in performance when exposed to high or low temperatures. For hikers, climbers, skiers and other outdoor recreationalists, cold temps are often the biggest challenge. To limit the effects that the cold has on battery power, try to keep your device warm. You can do this by keeping your headlamp, smartphone, GPS or other gadget somewhere close to your body.
- Do not attempt to simultaneously recharge batteries of different capacities, different brands or different ages. Do not use batteries of different brands or different ages together.
- Remove batteries from devices if they will be left unused for months at a time. This prevents a device from exerting a tiny drain on the batteries even though the device is inactive.
- Remove single-use (nonrechargeable) batteries from a device when they are being powered by household AC current. Doing so spares the batteries from any tiny drain on their power reserves by the device.
- Do not store batteries, particularly single-use batteries, in locations where heat can become intense, such as car trunks, attics or garages.
- Avoid tossing batteries into a drawer, briefcase or bag where they may contact metal objects such as coins or paper clips. Doing so may cause a short or it could negatively affect a battery's polarity.
- Never put batteries into a fire. Doing so could cause them to rupture and spill their contents. Also: Avoid tossing them into a metal container where heat could build up.










