9 disruptive battery technologies trying to compete with lithium-ion batteries
Silicon-based batteries
Li-ion batteries have traditionally used graphite anodes, but researchers and companies are now focusing on silicon anodes. The Si-dominant anodes can bind Li-ion 25-times more than the graphite ions. However, these batteries suffer from low electrical conductivity, a slow-diffusion rate and large volumetric fluctuations during lithiation. These limitations result in Si pulverization and instability of the solid electrolyte interphase (SEI).
Two primary strategies have been used to circumvent these challenges: nanotechnology and carbon coating. In the former method, various nano-sized Si anodes are used, which have a high surface area, improved cycle life and rate stability compared to bulk Si anodes. They can also withstand lithiation and delithiation without cracking. Carbon coating uses a combination of nanosized Si with different forms of carbon materials for generation of high-performance Si/C nanocomposite anodes.
Room-temperature sodium sulfur (RT-NaS) batteries
One of the most promising alternatives to lithium-sulfur batteries are sodium-sulfur batteries, due to similar physical and chemical properties of Na and Li ions. However, a high temperature (>300°C) is needed for battery operation. As a promising alternative, the low-cost RT-NaS battery system has generated extensive research interest for use in large-scale grid applications with enhanced safety. However, due to complex reactions within the battery, the RT-NaS batteries suffer from a lower theoretical capacity.
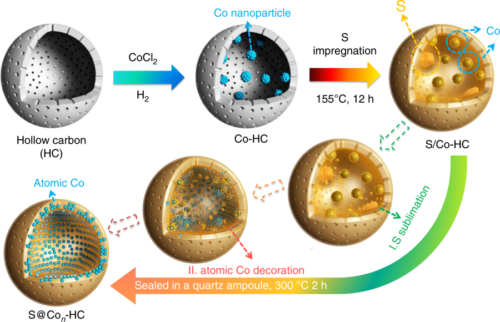
- Researchers at the University of Wollongong, Australia, focused on the electrode design. They built an efficient sulfur cathode with atomic Cobalt anchored in the micropores of hollow carbon nanospheres. The synthesized cathode demonstrated excellent electrochemical performance.
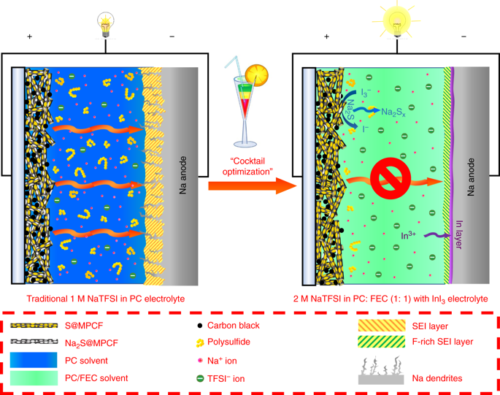
- In recent research published in “Nature,” scientists used a multifunctional carbonate electrolyte with high electrochemical performance and increased safety. This approach could be applied to a wide range of Na-based rechargeable battery systems for the advancement of low-cost and high-performance energy storage devices.
Though RT-NaS batteries are still in the early phase of development, companies like Ambri, a spin-out company from MIT led by Dr. Sadoway, is working to improve the battery design. The next generation of NaS-based energy storage technologies could soon become a reality with the ongoing research efforts and approaches discussed above.
Proton batteries
Graphite dual-ion batteries
- A team of researchers from various institutions discovered a novel family of honeycomb-layered compounds with a general formula of K2M2TeO6 (where M=Ni, Mg, Co, etc. or a combination of at least two transition metals). These honeycomb structured potassium-based tellurate compounds are suitable for high-voltage cathode materials and are capable of inserting K ions into ionic liquids, making them excellent candidates for the development of high-energy KIBs.
- Similarly, another team at the University of Wollongong developed a high-performance KIB with a composite of few-layered antimony sulfide/carbon sheet (SBS/C) anode.
- These novel approaches will help circumvent the limitations of the suitable host substrates for intercalating K ions and are a promising step towards attracting industrial investments for commercial applications.
- 7.Salt-water batteries
Many research efforts have been devoted to the generation of high-performance proton exchange membrane (PEM) fuel cells. However, the viability of PEM fuel cells has been a challenge due to their high cost, transportation and storage of hydrogen gas.
Dual-ion batteries (DIBs) that use metals other than lithium have attracted a lot of interest in recent years for large-scale stationary storage of electricity. Research efforts are on to increase the energy density of the DIBs by increasing the ionic content of the electrolyte and the ability of the electrodes to store charge
5.Nickel-zinc batteries
Nickel-zinc batteries are cost effective, safe, nontoxic, environment-friendly batteries that could compete with Li-ion batteries for energy storage. However, the main barrier for commercialization has been their low cycle life.
To address this problem, Chinese researchers from the Dalian University of Technology have developed a breakthrough in-situ cutting technique to improve the performance of Ni-Zn batteries by solving the issue of Zn electrode dissolution and suppressing the formation of dendrites. The team developed a novel graphene-ZnO hybrid electrode with the in-situ cutting technique, which can cut graphene directly into short nanoribbons. The strong interatomic interactions anchor Zn atoms onto graphene surfaces. This approach thoroughly fixes the issues of Zn electrode dissolution, dendrite formation, and performance.
6.Potassium-ion batteries
There have been a lot of recent breakthroughs to improve the electrochemical performance of potassium-ion batteries (KIBs). Three worth noting are listed below.
Water can conduct ions and be used to form rechargeable batteries. However, the chemical stability of water lasts up to 2.3 V, which is three times less than Lithium-ion batteries, limiting its use in EVs. These batteries could be suitable for stationary power-storage applications.
The salt-containing liquid has all the water molecules concentrated around the sodium cations in a hydrate shell, resulting in hardly any unbound water molecules present. This saline solution shows superior electrochemical stability of up to 2.6V, which is twice as high as other aqueous electrolytes. The prototype has shown promising results in the lab and can stand multiple charge-discharge cycles.
Although the current applications of the salt-water batteries are limited, they still offer several advantages, including safety, low cost and nontoxicity, for use in stationary storage systems.
8.Paper-polymer batteries
Paper-based microbial bio-batteries have generated widespread interest, as they are inexpensive, environment-friendly, and self-sustainable. They could have enormous applications in biosensors and future electronic devices. However, the main limitation is the low performance.
9.Magnesium batteries
Mg-based batteries could compete with Li-ion in theory, due to a higher energy density capacity. However, Mg-based batteries are not rechargeable, as the reversible reaction requires a corrosive electrolyte that creates a barrier for Mg2+ ions.










